

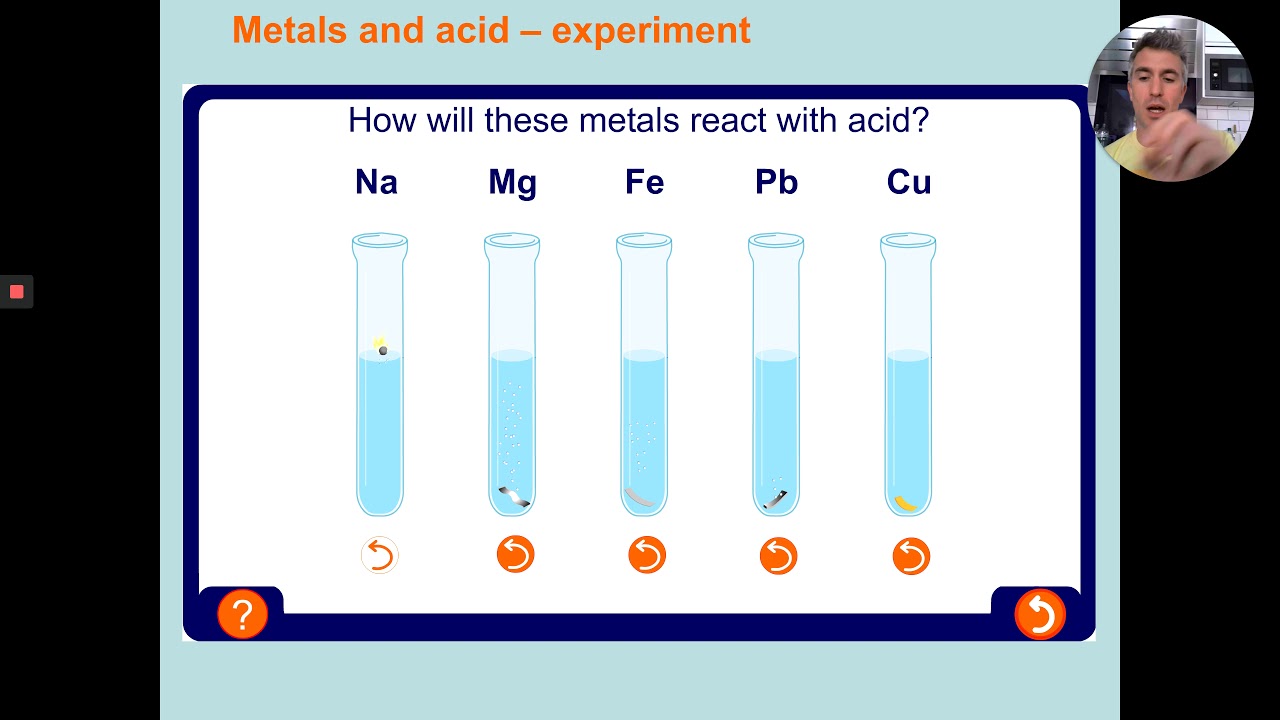
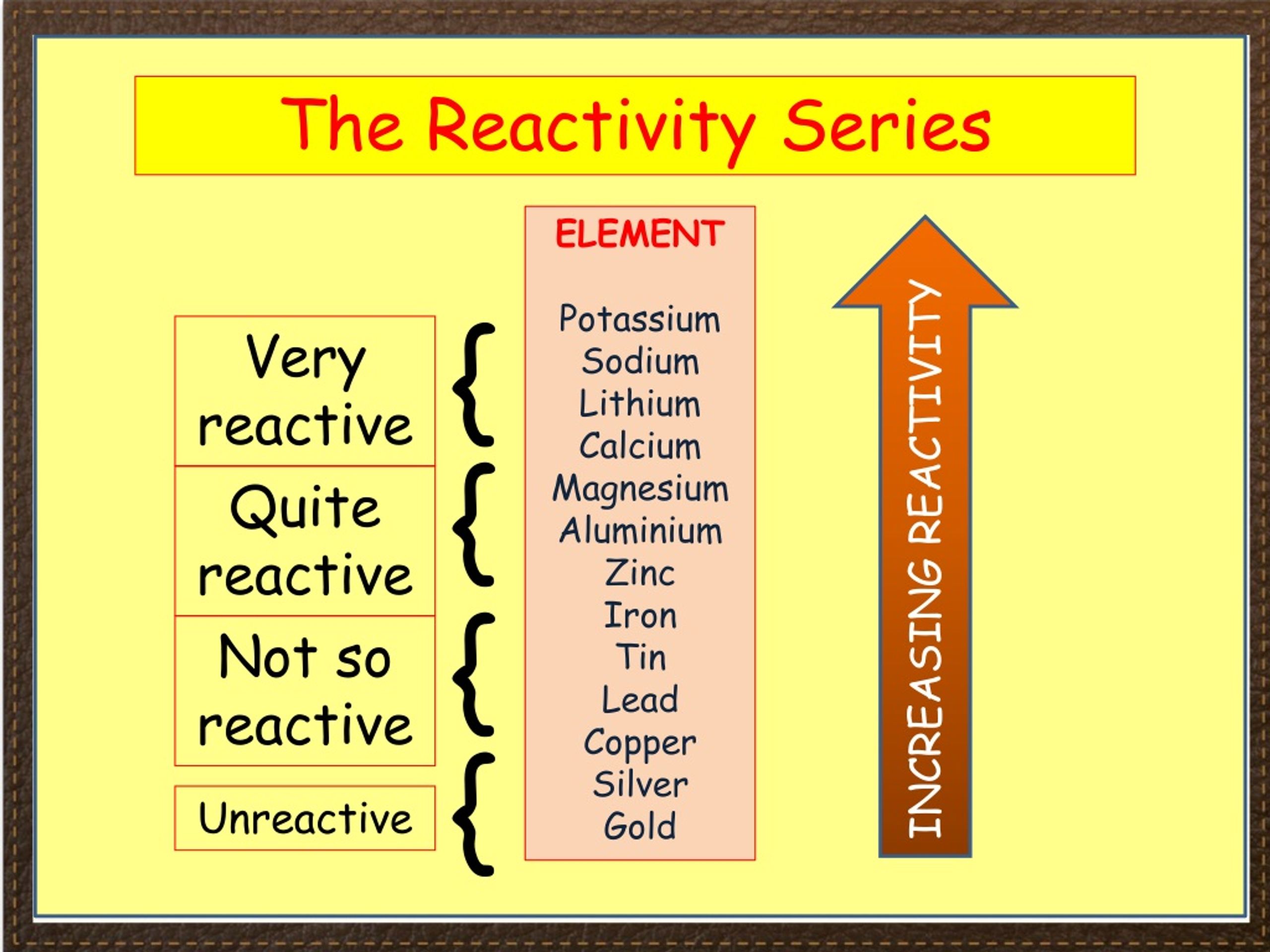
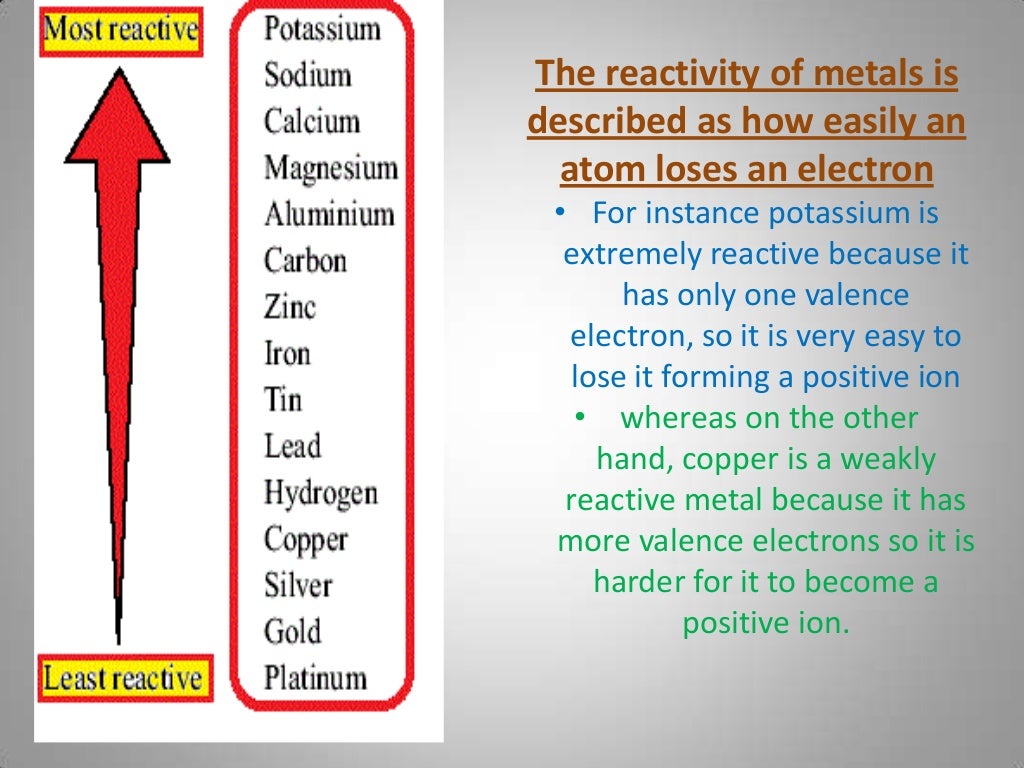
#Reactivity series practice how to#
1:28 understand how to carry out calculations involving amount of substance, relative atomic mass (Aᵣ) and relative formula mass (Mᵣ).1:27 know that the mole (mol) is the unit for the amount of a substance.1:26 calculate relative formula masses (including relative molecular masses) (Mᵣ) from relative atomic masses (Aᵣ).1:25 write word equations and balanced chemical equations (including state symbols): for reactions studied in this specification and for unfamiliar reactions where suitable information is provided.(e) Chemical formulae, equations and calculations.1:24 understand why the noble gases (Group 0) do not readily react.1:23 Understand why elements in the same group of the Periodic Table have similar chemical properties.1:22 understand how the electronic configuration of a main group element is related to its position in the Periodic Table.1:21 identify an element as a metal or a non-metal according to its position in the Periodic Table.1:20 understand how to use electrical conductivity and the acid-base character of oxides to classify elements as metals or non-metals.1:19 understand how to deduce the electronic configurations of the first 20 elements from their positions in the Periodic Table.1:18 understand how elements are arranged in the Periodic Table: in order of atomic number, in groups and periods.1:17 be able to calculate the relative atomic mass of an element (Aᵣ) from isotopic abundances.1:16 know what is meant by the terms atomic number, mass number, isotopes and relative atomic mass (Aᵣ).1:15 know the structure of an atom in terms of the positions, relative masses and relative charges of sub-atomic particles.1:14 know what is meant by the terms atom and molecule.1:13 practical: investigate paper chromatography using inks/food colourings.
1:12 understand how to use the calculation of Rf values to identify the components of a mixture.1:11 understand how a chromatogram provides information about the composition of a mixture.
1:10 describe these experimental techniques for the separation of mixtures: simple distillation, fractional distillation, filtration, crystallisation, paper chromatography.1:09 understand that a pure substance has a fixed melting and boiling point, but that a mixture may melt or boil over a range of temperatures.1:08 understand how to classify a substance as an element, a compound or a mixture.1:07 (Triple only) practical: investigate the solubility of a solid in water at a specific temperature.1:06 (Triple only) understand how to plot and interpret solubility curves.1:05 (Triple only) know what is meant by the term solubility in the units g per 100g of solvent.1:04 know what is meant by the terms: solvent, solute, solution, saturated solution.1:03 understand how the results of experiments involving the dilution of coloured solutions and diffusion of gases can be explained.1:02 understand the interconversions between the three states of matter in terms of: the names of the interconversions, how they are achieved and the changes in arrangement, movement and energy of the particles.1:01 understand the three states of matter in terms of the arrangement, movement and energy of the particles.


 0 kommentar(er)
0 kommentar(er)
